Quality
The Quality Management System is the Swiss Army Knife for the development of the biological resources and the know-how of a Biobank.
The biological samples collected, processed and stored in Biobanks are important research tools to perform efficient and high-quality research. Sample quality is a wide concept, which should be understood as a whole to establish standard control criteria and foster good biobanking practices.
« Having internationally agreed common guidelines helps to improve the performance of biobanks because it cuts through these differences and creates a worldwide shared understanding of quality, trust and reliability. »
Dr Ricardo Gent — Chair of the ISO committee
What means a Quality Management System (QMS) for a Biobank?
Control and monitoring of resources, respect for ethical and legal requirements, improvement policy and stakeholder trust. This QMS allows strategic orientation towards performance, to meet the needs of researchers and the expectations of society.
SBP has developed tools and support documents to help Biobanks in the establishment and implementation of their quality strategy. This documentation is at the cornerstone of quality and should be part of the Quality Control strategy developed by the biobank to satisfy the ISO 20387 standard requirement (“Biotechnology – Biobanking – General requirements for biobanking”).
What are the SBP labels?
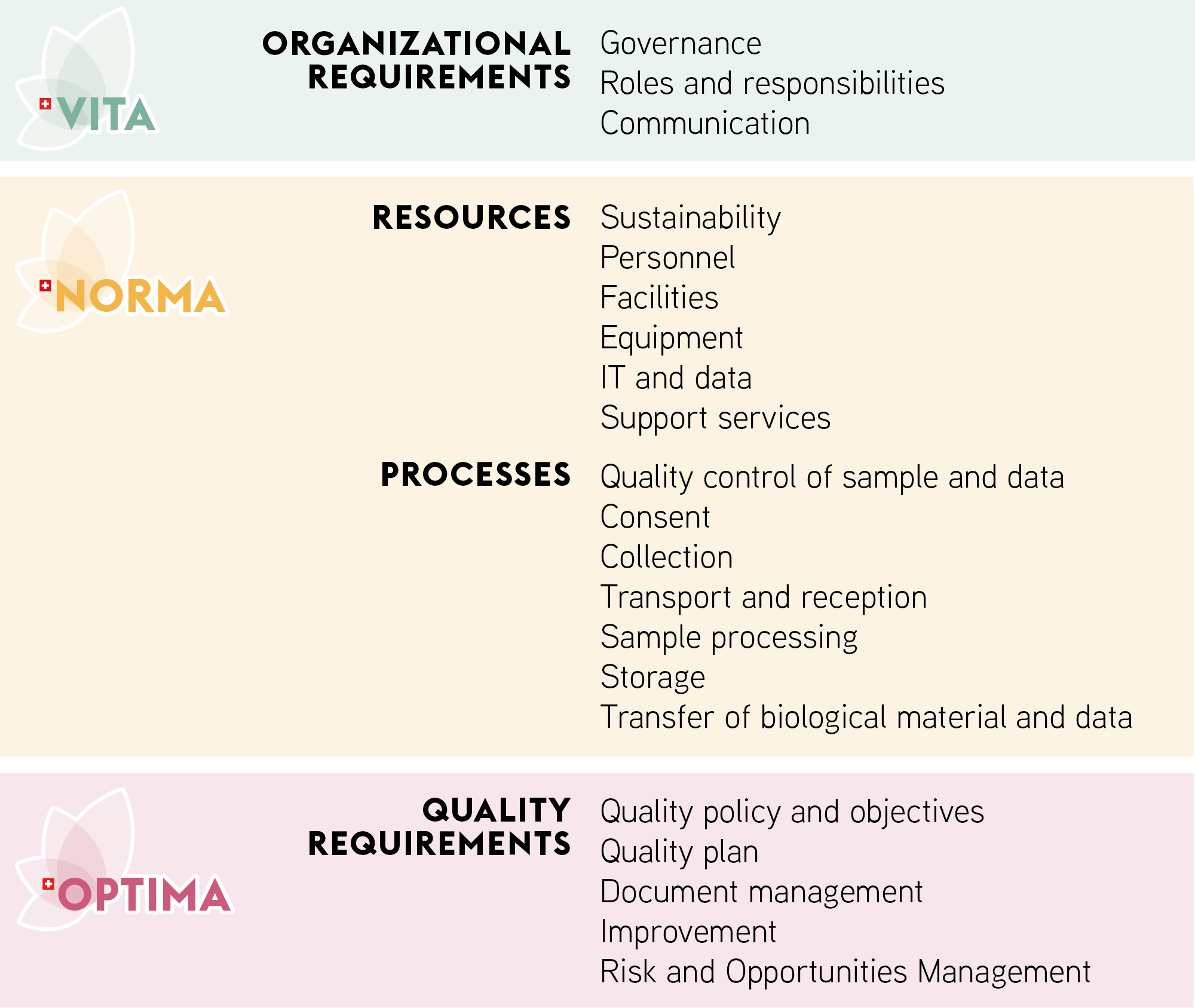
SBP proposes three Labels attesting good practice in biobanking
The SBP Labels have been launched in 2018 to distinguish the Biobanks’ compliance with the different minimal requirements and to proclaim their quality at a national level.
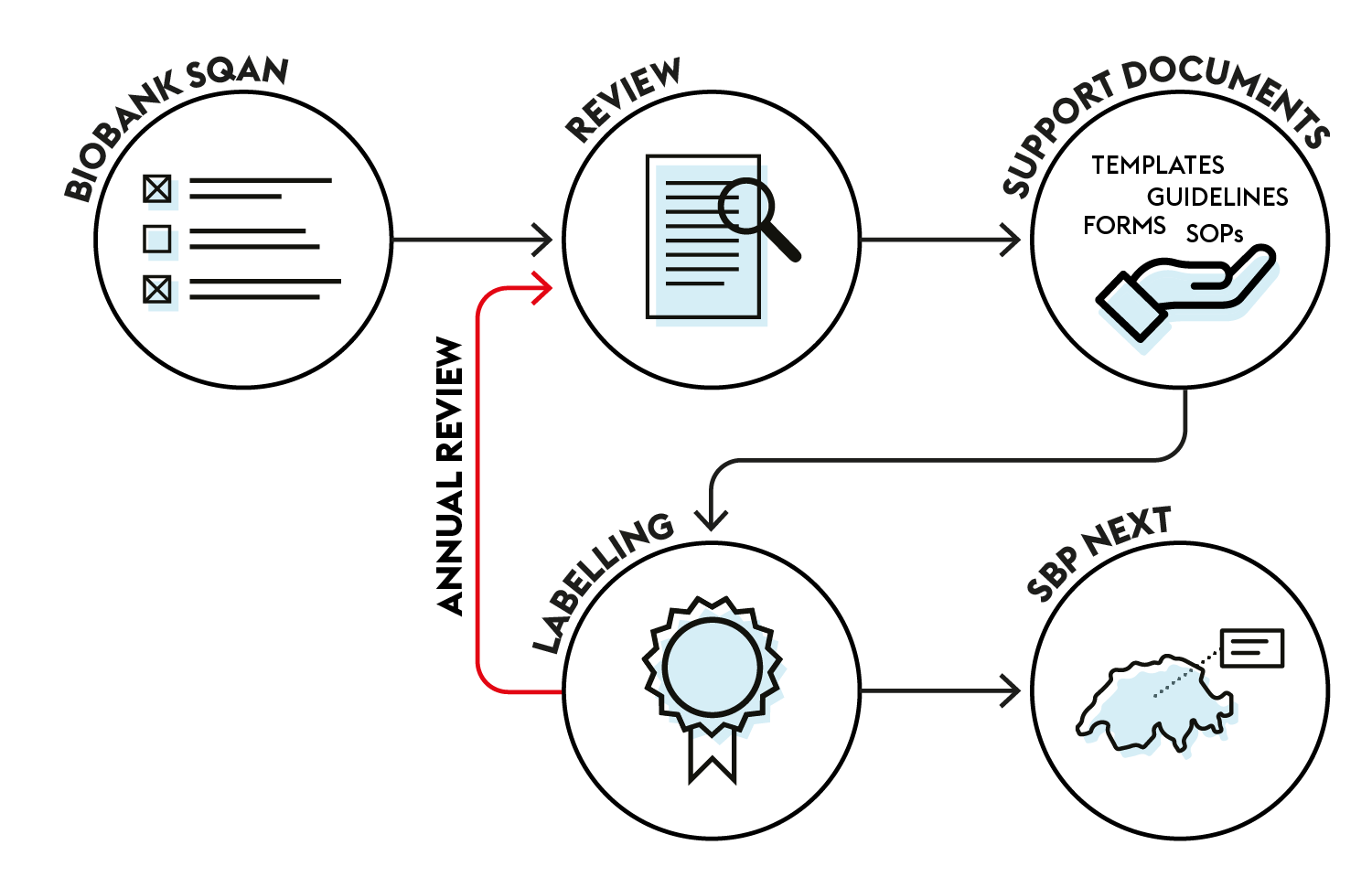
The compliance review is achieved through the Biobank SQAN, our online assessment tool for biobanking practice. After completion of the compliance review, the three labels can be awarded.
The VITA label evaluates the biobank compliance with the legal and ethical standards.
The NORMA label evaluates the biobank standardization of the multiple biobanking processes from consent presentation to sample shipment, including personnel and equipment management
The OPTIMA label evaluates the biobank optimization of the overall biobanking management system with focus on the implementation of quality assurance measures
How to obtain a label?
A clear process towards excellence
The Biobank SQAN is the interactive tool to support Biobanks and Biobank Infrastructures in the development of their governance and quality strategies.
Once registered, the Biobank takes the first step on the practice assessment ladder and will be supported by our experts to progressively obtain compliance with the following minimal requirements:
Ethical and legal issues for the VITA label
Operational issues for the NORMA label
Quality issues for the OPTIMA label
Each year, an annual update is conducted to validate the label(s).

Which labels do I need?
What your development strategy relies on
We provide support in every step of the way to make achieving your goal easy and ensure success.
SBP recommends its biobank partners to aim for at least for the NORMA label which will guarantee the quality of the biobanking processes and the harmonization of the biobanks’ practices at the national level. Efficient sample management is indeed a prerequisite to enable researchers to perform reliable and high-quality research.

SBP Quality Manual
A practical reference basis to lead biobanks towards biobanking Best Practices and International Quality Standards
The Quality Manual template is the reference deliverable guiding the biobank in the definition of its quality strategy. The template is designed to be straight to the point, comprehensive, adaptable and customizable for an easy implementation to each biobank’s practice making it a practical document supporting biobanks or biobank infrastructures to set up and document their quality management system (QMS). Describing all elements of a quality management system allows to have an efficient overview of the biobank operational procedures and helps identifying and addressing potential gaps.
This document is intended to be used internally as a reference document for the biobank personnel and externally for auditors, customers, and external collaborators.
Documentation of the quality management system is a requisite for the ISO 20387-General requirements for biobanking and for the obtention of a SBP OPTIMA Label, as the latter preparing biobanks to the ISO accreditation.